Originally published in Diver Magazine, Issue Dec 2008/Jan 2009, this article is Part I of a two part article on oxygen toxicity.
A common problem in diving is too much oxygen (hyperoxia). In this article I will review the mechanism of oxygen toxicity and in a follow up article I will review the signs and symptoms of oxygen toxicity.
Air is composed of 21% oxygen (O2). We require O2 to survive and without O2 we will die very quickly. Our bodies don’t actually care what percentage O2 we breathe, they respond to the partial pressure of O2 (pO2).
On the surface the partial pressure of O2 in air is 0.21 ATA (0.21 * 1.0 ATA = 0.21 ATA). As we saw in the last column, if we are young and healthy our bodies perform perfectly well at partial pressures of O2 down to 0.16 ATA and we can easily tolerate a pO2 of 0.12 ATA at rest. With chronic exposure we can adapt to even lower pO2s.
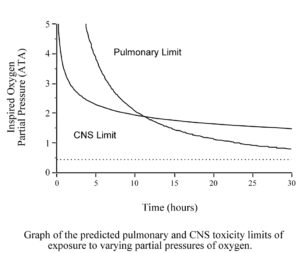
However, when we dive we are usually exposed too much higher pO2s. The human body is able to tolerate increased partial pressures of oxygen, up to about 0.45 ATA, without problem. When the pO2 rises above that level, toxic effects will eventually appear. The toxic effect of oxygen on the lungs is primarily a problem of long exposures (many hours or even days) to pO2s of between 0.45 and 1.6 ATA. At pO2s above 1.6 ATA, the toxic effects of oxygen on the brain occur (minutes to a few hours) before the toxic effects on the lungs.

Many recreational divers will not have to worry about oxygen toxicity because when diving air, the pO2 will never be high enough, for long enough, to cause problems. The narcotic effect of nitrogen causes air divers to limit their depth to a maximum of 130 fsw (40 msw). At that depth breathing air, the pO2 is just over 1.0 ATA, too low to worry about CNS toxicity. The limited size of our air supplies keeps bottom times short enough that we usually do not have to worry about lung toxicity.
However, many recreational divers are now diving Nitrox with up to 40% oxygen and some are using higher levels of oxygen or even pure oxygen for decompression. When you breathe higher percentages of oxygen, toxic effects are seen at shallower depths. The O2 in air does not reach a partial pressure of 1.6 ATA until a depth of 218 fsw (66 msw), far deeper than a recreational diver will go. However, the O2 in Nitrox40 will reach a pO2 of 1.6 ATA at a depth of only 99 fsw (30 msw), a depth most recreational divers will dive too on a regular basis. In addition, using Nitrox allows you to dive longer before requiring decompression stops, and to do shorter decompression stops if you get into decompression. As a result, recreational divers are using larger tanks, or multiple tanks, and doing longer dives. These longer dives also increase the risk of O2 toxicity. Therefore, all divers should have at least a basic understanding of oxygen toxicity.
Oxygen is a colourless, odourless, tasteless gas and makes up 20.98% of air by volume. The toxicity of oxygen is a function of the pO2, the time of exposure, and individual variation. There is a marked difference in the susceptibility of individuals to oxygen toxicity, and a change in the same individual from day to day.
The toxicity of oxygen is really a function of the pO2 in the cells and all cells will eventually die if they are exposed to a high enough pO2 for a long enough period of time. In living, breathing humans however, there are only two tissues that we need be concerned about, the lungs and the brain. The toxic effects of oxygen on these tissues will incapacitate us before the other tissues have a serious problem. To be perfectly correct, a third tissue can become a problem in rare instances where a rebreather diver has done a lot of diving, every day, for several days in a row. The eye can become near-sited. This ‘Hyperbaric Induced Myopia’ is beyond the scope of this column.
In general, the susceptibility of a cell to oxygen toxicity is related to its rate of metabolism. A resting cell is relatively resistant while an active cell is more susceptible.
This next point is critical to understanding oxygen toxicity. Normal oxygen is a molecule composed of two atoms of oxygen with a balanced number of protons and electrons so that the molecule does not have an electric charge. This normal molecule of oxygen is not toxic!!
The problem is that whenever molecular O2 exists, it forms other substances known as ‘oxygen radicals’. Oxygen radicals are highly reactive molecules, formed from oxygen, which often contain at least one extra electron. These molecules are formed from collisions between oxygen molecules, collisions between oxygen and other molecules, and as a result of metabolic processes in the cells. Examples include superoxide anions, hydrogen peroxide, hydroperoxy and hydroxyl radicals, and singlet oxygen. Oxygen radicals will often bind to the next molecule they come in contact with, usually damaging or changing that molecule. Therefore, whenever you have O2, you will have O2 radicals. Even if there was some magical way to remove all of the oxygen radicals from a tank of oxygen, more would immediately form. The number of O2 radicals is proportional to the partial pressure of O2.
There are hundreds of specific chemical reactions that oxygen radicals can be involved in that damage the cell, but in general terms there are three ways that they cause damage. The first is through inactivation of enzymes. Enzymes are proteins that work as catalysts, causing reactions to occur that would not normally occur at body temperature. They do this by holding the two molecules that are to react in exactly the right orientation to each other so that they join. The resulting molecule is released and the enzyme starts again, repeating the process thousands of times. If the shape of the enzyme is changed, the molecules will not be held in the right orientation and the reaction will not occur. Oxygen radicals cause cross-linking of sulphydryl groups, thereby changing the shape of the enzyme and inactivating it. They also cause changes in the shape of proteins responsible for transport of ions in and out of the cells across the cell membrane, stopping them from functioning. Finally, oxygen radicals cause peroxidation of the various lipids in the cells.
All cells in oxygen breathing animals have ways to inactivate oxygen radicals and to repair some of the damage done by them. The two main defenses are superoxide dysmutase and catalase. Both of these enzymes help maintain a good supply of reduced glutathione. Reduced glutathione has many sulphydryl groups and oxygen radicals will bind to them, and thus be unavailable to cause damage to the cell. Vitamins E and C are also anti-oxidants.
Oxygen radicals are not only important in diving, but are becoming very important in medicine. One of the methods white blood cells (WBC) use to kill bacteria is to enclose the bacteria in a membrane and then to inject oxygen radicals into the vacuole (the WBC makes the O2 radicals). The oxygen radicals actually kill the bacteria. In addition we now know that O2 radicals are the final method of damage in many diseases. Oxygen radicals are therefore both ‘good’ and ‘bad’.
It would seem reasonable to conclude that if O2 radicals cause cellular damage, taking ‘anti-oxidants’ should help reduce the damage. So far, the results of many well-designed studies have failed to show any benefit from taking anti-oxidant supplements. Some benefit has been shown when increased amounts of anti-oxidants are consumed by eating foods high in anti-oxidants. This suggests that something else in the food is required to get the beneficial effect of the anti-oxidants that is not available in the supplements.
The bottom line is that anytime O2 exists, O2 radicals will be formed. The number of O2 radicals is proportional to the pO2. All of our cells have defenses against the damage caused by O2 radicals. At normal pO2s, our cells are more than capable of repairing the damage being caused by the O2 radicals. As the pO2, and the number of O2 radicals is increased, a point is reached where the cells cannot repair the damage as quickly as it is occurring. Therefore, the damage will accumulate until the function of the cell is impaired or the cell dies.
Given the above explanation, it should be obvious that the toxicity of O2 will depend on the pO2 and the time of exposure. The other factor is that we are all biologically different and some individuals will have more defenses against O2 radicals than others. To further complicate the issue, our defenses against O2 radicals also change greatly from day to day. Therefore, we have marked differences in sensitivity to O2 radical damage in different people and on different days in the same person.
In the next article, I will discuss the effects of oxygen toxicity on the lungs and the brain.